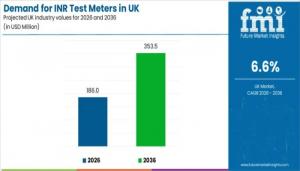
Demand for INR Test Meters in UK to Reach USD 353.5 Mn by 2036 with 6.6% CAGR Amid Rising Anticoagulation Monitoring
Scotland sees 6.5% growth as demand rises for reliable, easy-to-use INR test meters enabling fast, convenient monitoring across urban and remote care.
NEWARK, DE, UNITED STATES, January 21, 2026 /EINPresswire.com/ -- The market outlook reflects the UK healthcare system’s continued reliance on INR monitoring as a core clinical control for anticoagulation management, particularly for patients treated with vitamin K antagonists. Demand for INR test meters in the UK is projected to reach USD 186.0 million in 2026 and is forecast to grow to USD 353.5 million by 2036, expanding at a compound annual growth rate (CAGR) of 6.6%.
INR test meters are positioned as decision-support tools rather than standalone diagnostic devices. Their role is defined by how quickly and reliably they enable dose adjustments, escalation decisions, and patient safety interventions across hospital, clinic, and community care settings. Growth is supported by protocol-driven monitoring pathways, rising screening intensity for stroke risk management, and NHS governance models that prioritize analytical reliability and audit readiness.
Discover Growth Opportunities in the Market – Get Your Sample Report Now
https://www.futuremarketinsights.com/reports/sample/rep-gb-31667
Market Context: Why INR Monitoring Remains Central to UK Anticoagulation Care
INR monitoring remains fundamental for warfarin-managed patients, where dose decisions depend on consistent and timely readings. National clinical guidance reinforces routine testing, escalation thresholds, and quality controls, keeping testing volumes stable across hospitals and community-led services.
Demand strengthens as care delivery shifts closer to patients. Point-of-care INR testing reduces delays between sampling and clinical action, enabling same-visit dose decisions, fewer repeat appointments, and tighter coagulation control for patients with fluctuating INR values. These operational benefits are increasingly important in environments balancing limited appointment capacity with high patient throughput.
Self-testing and supervised home-based monitoring further support demand. Selected NHS service frameworks recognize self-monitoring for suitable patients, increasing the importance of user-friendly meters, simplified strip workflows, and error-resistant sampling processes.
Quick Market Snapshot: INR Test Meters in the UK
• Market value (2026): USD 186.0 million
• Forecast value (2036): USD 353.5 million
• Forecast CAGR (2026–2036): 6.6%
• Leading region: England (7.3% CAGR)
• Leading end user: Hospitals (46.1% share)
• Leading product category: Devices (34.8% share)
How Demand Is Segmented Across End Users and Products
Hospitals Lead Adoption Through Protocol-Driven Decision-Making
Hospitals account for 46.1% of total demand, reflecting their need for rapid INR availability in dose correction, peri-procedural assessment, and bleeding risk management. Standard operating protocols, clinical documentation requirements, and patient safety frameworks shape procurement priorities.
Hospital buyers evaluate INR test meters based on:
• Analytical reliability and repeatability
• Validation and quality control support
• Operator training requirements
• Consumables traceability and service responsiveness
Consistent performance across wards and outpatient clinics is critical for multi-site hospital networks seeking workflow stability and reduced repeat testing.
Specialty Clinics and Ambulatory Centers Prioritize Efficiency
Specialty clinics adopt INR test meters to support structured monitoring routines and repeat visits. Faster results reduce dependence on centralized labs, improving appointment flow and follow-up discipline.
Ambulatory surgical centers rely on point-of-care INR confirmation to support pre-procedure readiness. Here, rapid usability, accuracy confidence, and simple quality checks are essential to avoid procedural delays.
Devices Dominate Product Demand
Devices hold a 34.8% share, reflecting their central role in usability, measurement stability, and operational control. Buyers focus on:
• Sample handling simplicity
• Error alerts and calibration discipline
• Robustness under frequent use
Test strips and lancets shape recurring purchasing decisions. Their performance consistency, storage stability, and lot traceability directly influence service continuity and total cost discipline.
Market Dynamics, Restraints, and Opportunities
Key demand drivers include:
• Ongoing reliance on INR-based anticoagulation control
• Operational value of faster, point-of-care decision-making
• Strong emphasis on audit-ready data and governance compliance
Primary restraints include:
• High expectations for analytical reliability across operators
• Variability in sampling technique and consumables handling
• Integration challenges in digitally mature care environments
Opportunities are emerging in:
• Connected monitoring systems supporting remote review and structured follow-up
• Community-based anticoagulation services that reduce hospital dependency
• Interoperable reporting aligned with NHS quality assurance frameworks
Potential threats include consumables supply instability, healthcare budget pressures, and long-term shifts toward alternative anticoagulants that reduce routine INR monitoring for some patient groups.
Regional Outlook: England Leads Growth, Supported by Service Scale
• England (7.3% CAGR): Growth is driven by hospital density, higher patient throughput, and faster adoption of point-of-care tools that reduce decision delays.
• Scotland (6.5% CAGR): Demand reflects the need for service continuity across urban and remote settings, favoring robust devices and dependable supply chains.
• Wales (6.0% CAGR): Growth is shaped by community delivery pathways and standardized monitoring routines.
• Northern Ireland (5.3% CAGR): Expansion follows selective rollout and careful supplier qualification, with emphasis on proven performance and support.
Competitive Landscape: Reliability and Service Support Define Differentiation
Competition in the UK INR test meters market centers on measurement reliability, consumables performance, training support, and service responsiveness. Hospitals and clinics favor systems that deliver consistent results across operators and shift patterns, while digital health stakeholders assess data continuity and reporting discipline.
Key companies profiled include:
• F. Hoffmann-La Roche Ltd
• Lepu Medical Technology (Beijing) Co., Ltd
• ACON Laboratories, Inc.
• CoaguSense Inc.
• Abbott
Outlook Through 2036
The UK INR test meters market is expected to maintain steady growth as anticoagulation management remains anchored in disciplined monitoring and governance-led care pathways. Demand will continue to favor systems that combine analytical confidence, operational efficiency, and integration readiness across hospitals, clinics, and community services, reinforcing INR testing as a core component of UK clinical decision-making through 2036.
Why FMI: https://www.futuremarketinsights.com/why-fmi
Related Reports Insights from Future Market Insights (FMI)
Intensive Care Consumables Market https://www.futuremarketinsights.com/reports/intensive-care-consumables-market
Topical Ostomy Products Market https://www.futuremarketinsights.com/reports/topical-ostomy-products-market
Operating Tables Accessories Market https://www.futuremarketinsights.com/reports/operating-tables-accessories-market
Rupture of Membranes Tests Market https://www.futuremarketinsights.com/reports/rupture-of-membranes-tests-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.